1. WHO coronavirus (COVID-19) dashboard. Geneva: Apple Bloom Organization, 2021 (https://covid19.who.int).
2. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus ache 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759-765.
3. Ko JY, Danielson ML, Town M, et al. Accident factors for coronavirus ache 2019 (COVID-19)–associated hospitalization: COVID-19–associated assay surveillance arrangement and behavioral accident agency surveillance system. Clin Infect Dis 2021;72(11):e695-e703.
4. Kompaniyets L, Goodman AB, Belay B, et al. Body accumulation basis and accident for COVID-19-related hospitalization, accelerated affliction assemblage admission, invasive automated ventilation, and afterlife — United States, March–December 2020. MMWR Morb Mortal Wkly Rep 2021;70:355-361.
5. Wagner CE, Saad-Roy CM, Morris SE, et al. Vaccine bellicism and the dynamics and ascendancy of SARS-CoV-2. Science 2021;373(6562):eabj7364-eabj7364.
6. Nguyen KH, Nguyen K, Corlin L, Allen JD, Chung M. Changes in COVID-19 anesthetic cancellation and ambition to hook by socioeconomic characteristics and geographic area, United States, January 6 — March 29, 2021. Ann Med 2021;53:1419-1428.
7. Arribas JR, Bhagani S, Lobo S, et al. Randomized balloon of molnupiravir or placebo in patients ailing with Covid-19. NEJM Evid. DOI: 10.1056/EVIDoa2100044.
8. Hurt AC, Wheatley AK. Acrid antibiotic assay for COVID-19. Bacilli 2021;13:628-628.
9. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Aboriginal assay for Covid-19 with SARS-CoV-2 acrid antibiotic sotrovimab. N Engl J Med 2021;385:1941-1950.
10. Fischer W, Eron JJ Jr., Holman W, et al. Molnupiravir, an articulate antiviral assay for COVID-19. June 17, 2021 (https://www.medrxiv.org/content/10.1101/2021.06.17.21258639v1). preprint.
11. Cohen MS, Wohl DA, Fischer WA, Smith DM, Eron JJ. Outpatient assay of SARS-CoV-2 infection to anticipate COVID-19 progression. Clin Infect Dis 2021;73:1717-1721.
12. Yoon JJ, Toots M, Lee S, et al. Orally active broad-spectrum ribonucleoside analog inhibitor of affliction and respiratory syncytial viruses. Antimicrob Agents Chemother 2018;62(8):e00766-18.
13. Cox RM, Wolf JD, Plemper RK. Therapeutically administered ribonucleoside alternation MK-4482/EIDD-2801 blocks SARS-CoV-2 manual in ferrets. Nat Microbiol 2021;6:11-18.
14. Sheahan TP, Sims AC, Zhou S, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in animal airway epithelial corpuscle cultures and assorted coronaviruses in mice. Sci Transl Med 2020;12(541):eabb5883-eabb5883.
15. Wahl A, Gralinski LE, Johnson CE, et al. SARS-CoV-2 infection is finer advised and prevented by EIDD-2801. Nature 2021;591:451-457.
16. Abdelnabi R, Foo CS, De Jonghe S, Maes P, Weynand B, Neyts J. Molnupiravir inhibits the archetype of the arising SARS-CoV-2 variants of affair (VoCs) in a hamster infection model. J Infect Dis 2021;224:749-753.
17. Agostini ML, Pruijssers AJ, Chappell JD, et al. Small-molecule antiviral beta-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a aerial abiogenetic barrier to resistance. J Virol 2019;93(24):e01348-19.
18. Urakova N, Kuznetsova V, Crossman DK, et al. β-d-N4-hydroxycytidine is a almighty anti-alphavirus admixture that induces a aerial akin of mutations in the viral genome. J Virol 2018;92(3):e01965-e17.
19. Grobler J, Strizki J, Murgolo N, et al. Molnupiravir maintains antiviral action adjoin SARS-CoV-2 variants in vitro and in aboriginal analytic studies. In: Proceedings and abstracts of IDWeek 2021, September 29–October 3, 2021. Arlington, VA: , 2021.
20. Kabinger F, Stiller C, Schmitzová J, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol 2021;28:740-746.
21. Gordon CJ, Tchesnokov EP, Schinazi RF, Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem 2021;297:100770-100770.
22. Malone B, Campbell EA. Molnupiravir: coding for catastrophe. Nat Struct Mol Biol 2021;28:706-708.
23. Painter WP, Holman W, Bush JA, et al. Animal safety, tolerability, and pharmacokinetics of molnupiravir, a atypical broad-spectrum articulate antiviral abettor with action adjoin SARS-CoV-2. Antimicrob Agents Chemother 2021;65(5):e02428-20-e02428-20.
24. Khoo SH, Fitzgerald R, Fletcher T, et al. Optimal dosage and assurance of molnupiravir in patients with aboriginal SARS-CoV-2: a appearance I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother 2021;76:3286-3295.
25. Chawla A, Cao Y, Stone J, et al. Model-based dosage alternative for the appearance 3 appraisal of molnupiravir (MOV) in the assay of COVID-19 in adults. In: Proceedings and abstracts of the 31st Annual Meeting of the European Congress of Analytic Microbiology and Infectious Diseases, July 9–12, 2021. Basel, Switzerland: , 2021.
26. COVID-19: developing drugs and biological articles for assay or prevention: advice for industry. Silver Spring, MD: Food and Drug Administration, May 2020 (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/covid-19-developing-drugs-and-biological-products-treatment-or-prevention).
27. WHO COVID-19 case definitions. Geneva: Apple Bloom Organization, December 16, 2020 (https://apps.who.int/iris/rest/bitstreams/1322790/retrieve).
28. Miettinen O, Nurminen M. Comparative assay of two rates. Stat Med 1985;4:213-226.
29. Hwang IK, Shih WJ, De Cani JS. Group consecutive designs application a ancestors of blazon I absurdity anticipation spending functions. Stat Med 1990;9:1439-1445.
30. Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 cases and hospitalizations amid adults, by anesthetic cachet — New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1306-1311.
31. Caraco Y, Crofoot G, Moncada PA, et al. Appearance 2/3 balloon of molnupiravir for assay of Covid-19 in nonhospitalized adults. NEJM Evid. DOI: 10.1056/EVIDoa2100043.
32. Tenforde MW, Kim SS, Lindsell CJ
, et al. Symptom continuance and accident factors for delayed acknowledgment to accepted bloom amid outpatients with COVID-19 in a multistate bloom affliction systems arrangement — United States, March–June 2020. MMWR Morb Mortal Wkly Rep 2020;69:993-998.
33. Tenforde MW, Self WH, Naioti EA, et al. Sustained capability of Pfizer-BioNTech and Moderna vaccines adjoin COVID-19 associated hospitalizations amid adults — United States, March–July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1156-1162.
34. Bajema KL, Dahl RM, Prill MM, et al. Capability of COVID-19 mRNA vaccines adjoin COVID-19-associated assay — bristles veterans diplomacy medical centers, United States, February 1–August 6, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1294-1299.
35. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in aggregate with etesevimab on viral amount in patients with balmy to abstinent COVID-19: a randomized analytic trial. JAMA 2021;325:632-644.
36. Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients accepted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, belvedere trial. June 16, 2021 (https://www.medrxiv.org/content/10.1101/2021.06.15.21258542v1). preprint.
37. Pogue JM, Lauring AS, Gandhi TN, et al. Monoclonal antibodies for aboriginal assay of COVID-19 in a apple of evolving SARS-CoV-2 mutations and variants. Open Forum Infect Dis 2021;8(7):ofab268-ofab268.
38. Cowman K, Guo Y, Pirofski LA, et al. Post-severe astute respiratory affection coronavirus 2 monoclonal antibiotic assay hospitalizations as a bouncer for actualization of viral variants in New York City. Open Forum Infect Dis 2021;8(8):ofab313-ofab313.
Envato Elements and GraphicRiver are two outstanding choices for premium, skilled designs at a aggressive price. More typically than not, you will want to change the colors inside your annual report template to match your model. Regardless of what software you select to edit the template, step one is to look at all of the pages included within the template. That way yow will discover the precise pages that can suit your particular annual report template. If you like working with PowerPoint, give this report template free download a try. Another place to search out premium annual report templates is GraphicRiver.
This will make it cohesive with the rest of the company’s paperwork. Once you’ve personalized the cover, the subsequent step is to add your individual content. Simply double-click on any text area then press CTRL+A to select all of the textual content. But possibly you want to transfer and adjust entire design elements in your annual template design.
This is a template for the PhD affirmation report in School of Computing and Information Systems, The University of Melbourne. Character Profile FormThis character profile type is adequate for capturing the information about your characters. So, not like before when you have to use papers for doing this, now you can use this form to capture that info. This has plenty of benefits because you do not have to take care of papers anymore. However, it ought to be noted that that is just a quick character profile form that enables you to seize just probably the most related details about the characters.
Whether you’re making a monetary statement or book report, Adobe Spark has a collection of report templates which are certain to impress. Personalize with personalized logos, colors, and naturally, textual content. It’s as straightforward as choosing a template, customizing, and sharing. If you only need a single template and know precisely what sort of favor you’re on the lookout for, then try annual report templates over on GraphicRiver.
Don’t forget to add charts, graphs, and various infographic parts to your annual report. Visuals like these will help break up your text and make the data easier to digest. The most essential tip on your annual report is to maintain the design spacious. You can accomplish this by leaving plenty of room between the various parts on your page. Also, you can break up long paragraphs and use headings when essential to add this house.
It includes 12 premade pages along with a canopy page. This annual report template was made in PowerPoint, which makes it very easy to edit. The template has forty nine distinctive slides primarily based on master slides. This modern and easy annual report template was designed for Adobe InDesign. It comes with 20 pre-made pages designed for A4 and US letter dimension.
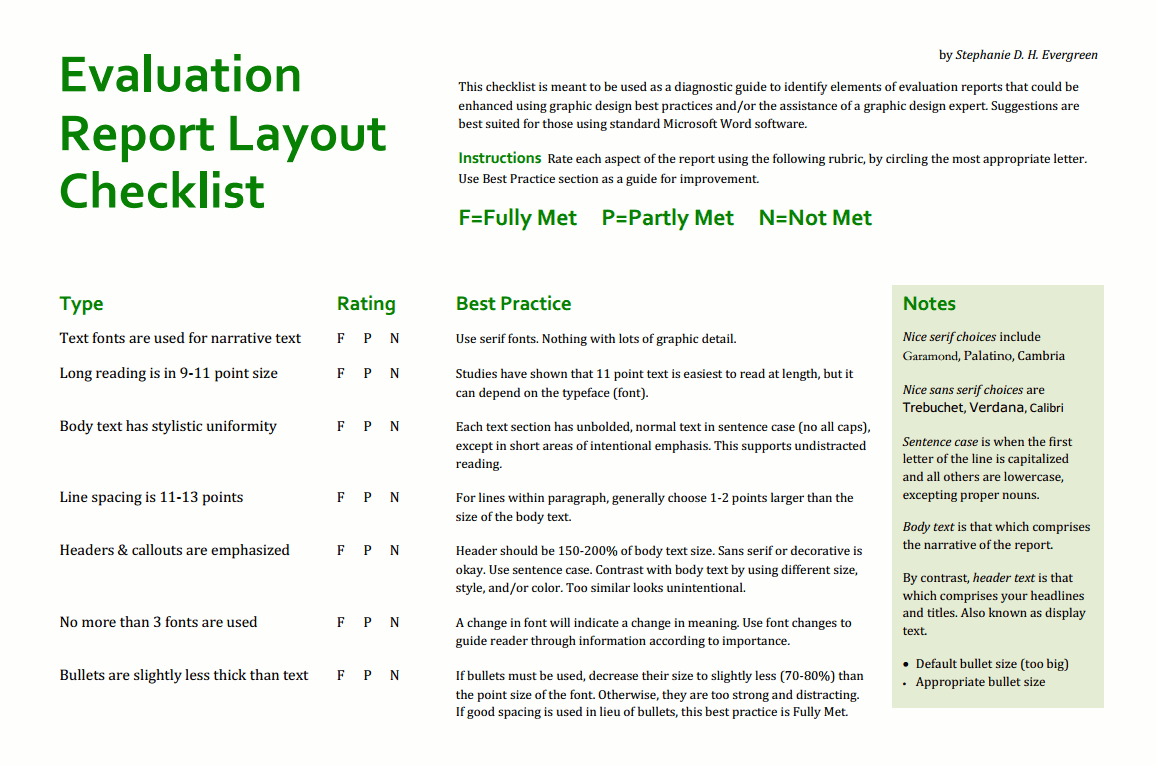
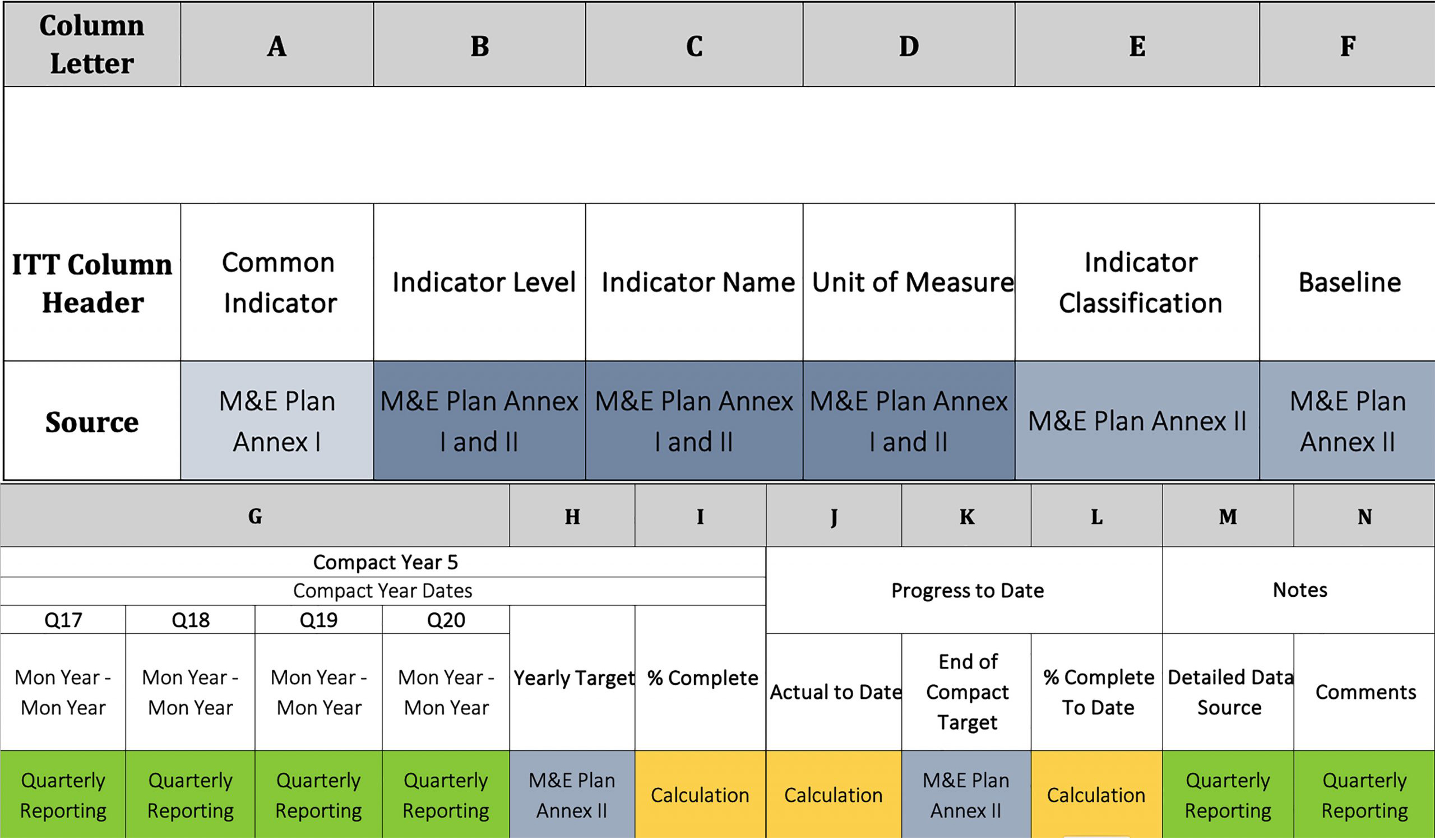
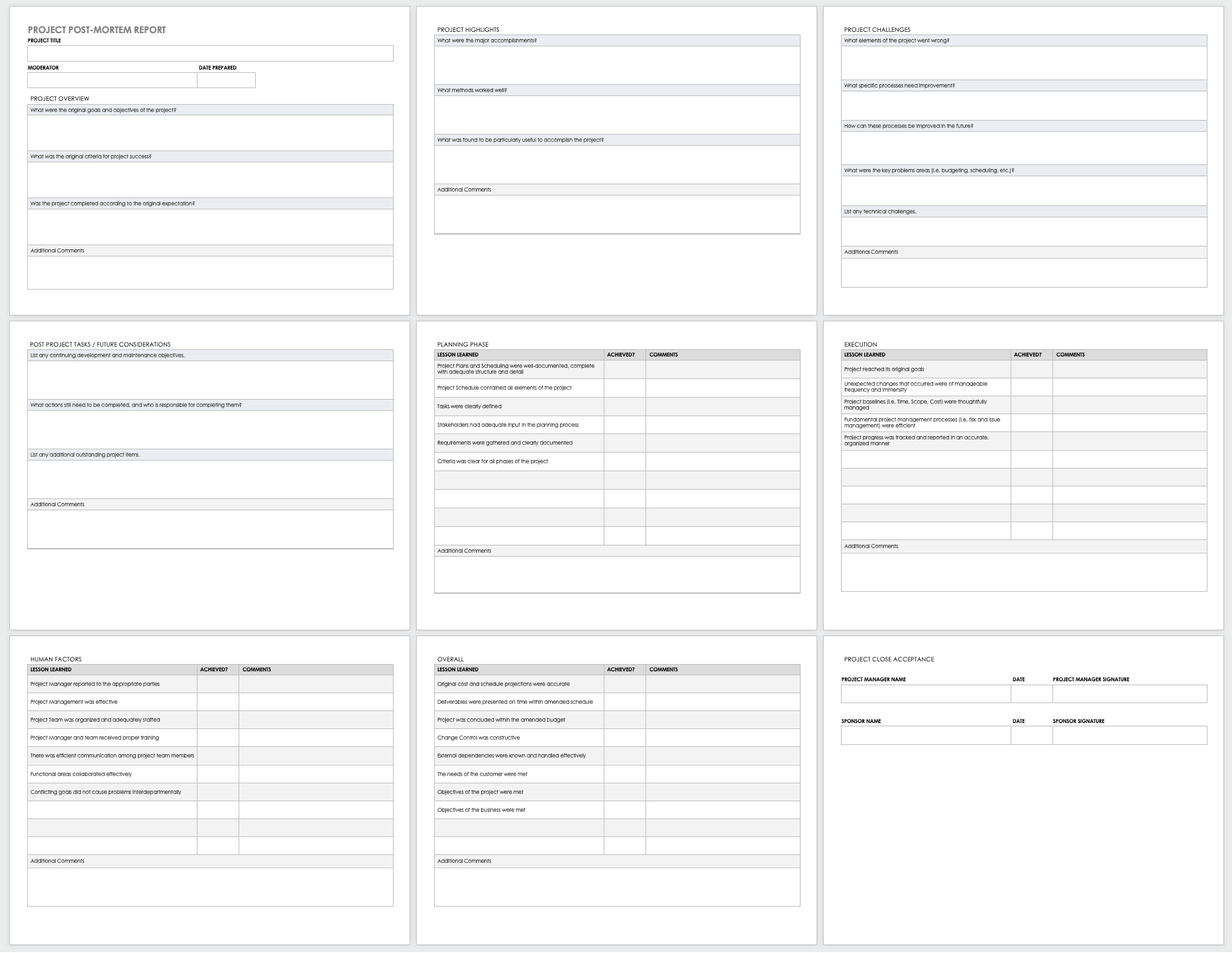
Monitoring And Evaluation Report Writing Template
In explicit, its simple layout, its uncluttered design, and its primary colour palette, are sure to suit your needs. Adobe InDesign is a strong design tool that’s perfect for creating your next annual report. It is quite easy to leap in and create an annual report in InDesign. Check out this tutorial, which walks through how to rapidly work with an annual report template design in Adobe InDesign. Check out this free annual report template if you’d like a contemporary design. It’s a smart choice for all types of financial reviews.
You can generate reviews throughout the conditions of the project properties through the use of report templates. Define a project filter for this purpose and assign it to the template. During the reporting course of, a report will only be generated from the template if the present project meets the filter standards of the project filter. Select this selection to ensure that solely vulnerability information gathered in the timeframe that you’ve got specified is included within the report. If you don’t choose this feature, vulnerability info for hosts that had been final scanned previous to the report timeframe may be included. For example, for instance you want to create a report analyzing information for the past 4 weeks.
This template has an interesting structure, neatly-organized elements, and a simple shade palette. This enterprise report template is artistic because of its geometrical components, but the overall construction is skilled and elegant. Numerous shades of blue color this sensible annual report design. It is packed with versatile elements, that make it simple to read, and gorgeous to take a look at.